Research
During my graduate research with Dr John Tyson, I built mathematical models to understand dynamical aspects:
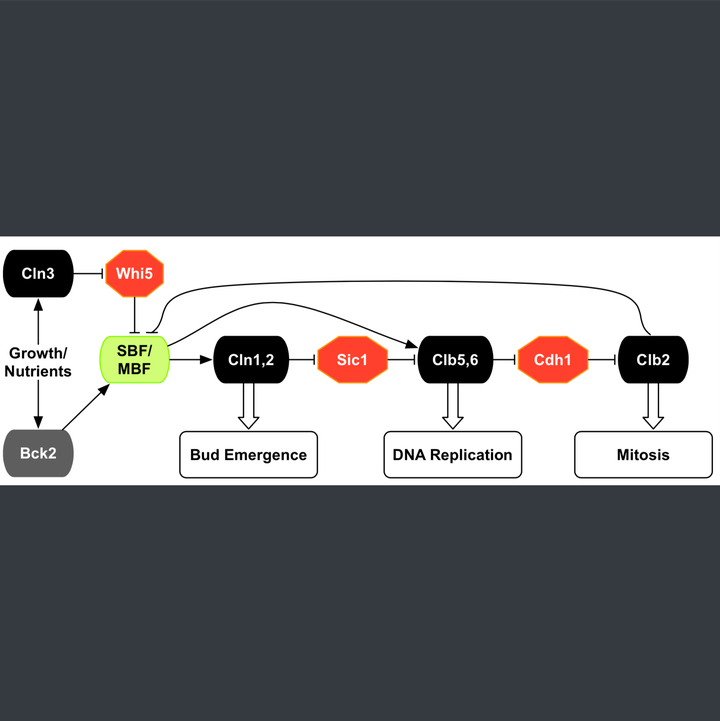
Modeling the START transition in the budding yeast cell cycle:
- Built a detailed mathematical model (~100 ODEs, ~150 parameters) for the START transition in yeast and integrated it with our published model of the whole cell cycle.
- Model addresses outstanding issues related to the precise mechanism and timing of transcriptional, post- translational and localization events, as well as size control under varying growth conditions.
- Model consistent with ~200 experimental mutant phenotypes pertaining to the START transition and rest of the cell cycle.
- Built a basic model for the nutritional effect of size control in budding yeast cells.
- This mechanism has been incorporated into the existing model of the yeast cell cycle to explain an initial set of START mutants.
Modeling bistability in the canonical Wnt pathway:
- Built a simplified model based upon the core module of the Wnt canonical pathway, and incorporated additional key regulatory interactions.
- Model shows that the Wnt signaling pathway can display bistability, in agreement with preliminary experimental results.
Related publications
- Ravi J, Tyson JJ. Modeling the START transition in the budding yeast cell cycle. Submitted.
- Thorne C, Ravi J, Tyson JJ, Lee E. Modeling bistability in the canonical Wnt signaling pathway. Submitted.
Collaborators
Virginia Tech
- Kathy Chen (now retd.)
Vanderbilt University
- Ethan Lee
- Curtis A. Thorne (now at University of Arizona)